Mount Sinai School of Medicine
-
CG Life is a precision medicine launch agency that’s at the forefront of crafting compelling narratives for Life Science companies navigating the drug discovery process.
During the interview process for a Strategic Communications Intern position, I was tasked with a communications exercise. My job? To translate scientific findings through a press release, secure earned media coverage, and create LinkedIn social content. -
Write a clear, engaging press release that shines a spotlight on the enzyme therapy for a general audience
Develop a compelling media narrative tailored towards a leading voice in biotech/biopharma
Craft LinkedIn content that effectively communicates the therapy’s relevance to patients, healthcare providers, and investors
Demonstrate storytelling that bridges science and strategy, in alignment with Icahn’s brand- a research institution pioneering scientific innovation
-
Brief Review - Analyzed CG Life brief containing key information - from clinical trial to Icahn scientific platforms
Scientific Translation - Next, I drafted a clear and concise press release that built excitement for positive topline data while featuring quotes from scientific leaders
Picking the right storyteller - I used MuckRack to find a reporter whose previous coverage aligned with the story and pitch
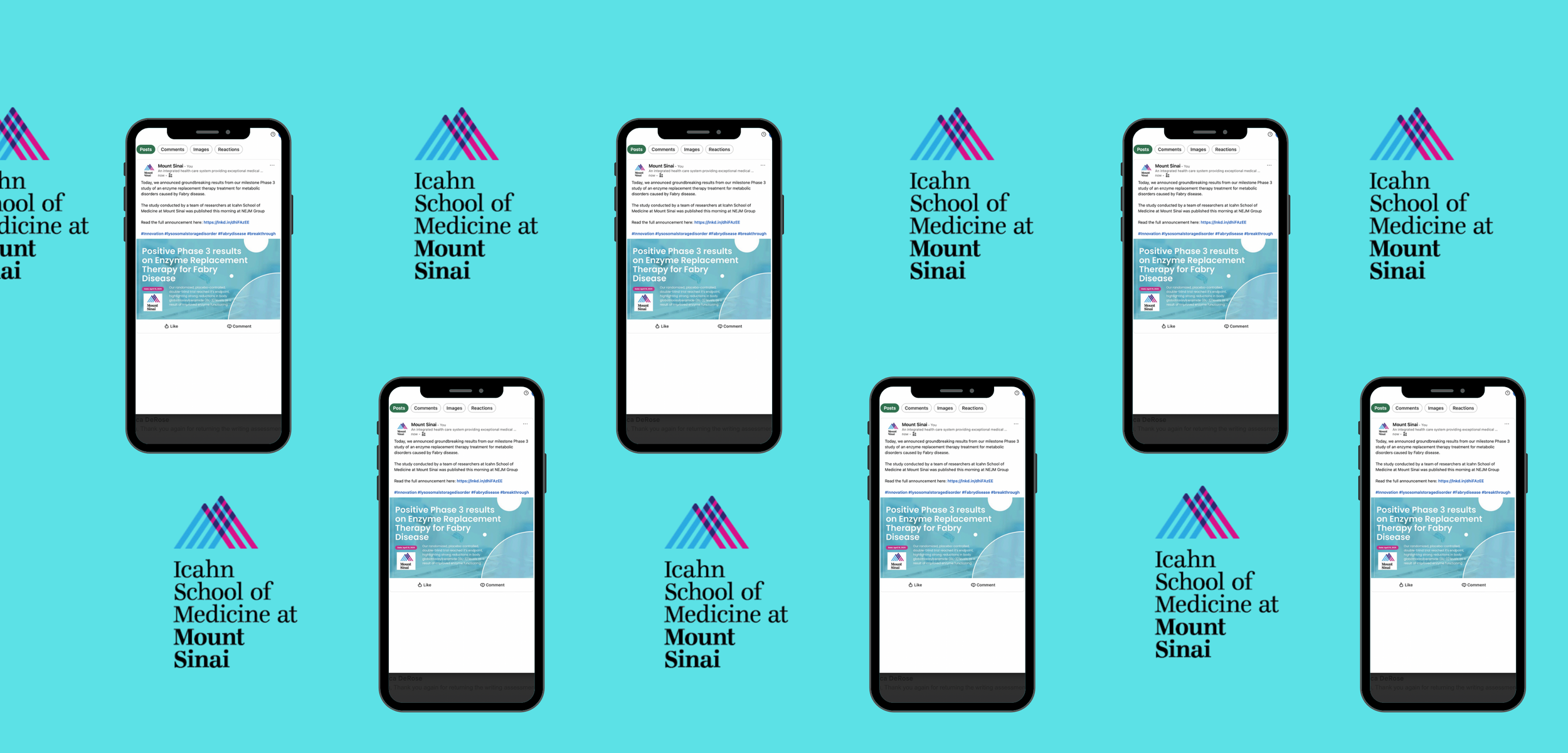
Crafting a campaign with reach - Put together a media pitch to win coverage around the release and designed sharp visuals + copy for LinkedIn that would reach key Icahn stakeholders
-
This communications exercise gave me hands-on experience diving into the world of precision medicine and fusing digital and traditional media strategy for key announcements. I learned how to research key media targets, frame messaging differently for media, investors, and patients, and strike a balance between scientific accuracy and public understanding- all crucial skills in life science storytelling.
The end product got me a second-round interview which eventually led me to being hired as a Strategic Communications intern at CG Life
PRESS RELEASE
NEW YORK, N.Y., April 15, 2025 --- Today, researchers at Icahn School of Medicine published results from a Phase 3 clinical study that demonstrates the effectiveness of an enzyme replacement therapy treatment to combat malfunctioning enzymes resulting from Fabry disease. Published in the New England Journal of Medicine, the study was the largest ever undertaken for Fabry disease and was conducted by a team of researchers led by Dr. Robert J. Desnick at Mount Sinai's East Harlem hospital. The multi-center, randomized, placebo-controlled, double-blind trial intended to test the efficacy of an enzyme-replacement therapy's ability to reverse metabolic defects associated with Fabry disease.
"We are dedicated to advancing transformative therapies that address the root causes of rare genetic disorders like Fabry disease" said Kenneth L. Davis, M.D., Executive Vice Chairperson of the Board of Trustees, Icahn School of Medicine. "These high-quality results show that the enzyme replacement therapy has the potential to combat lysosomal storage disorders by reducing GL-3 levels in patients with malfunctioning enzymes. We hope to continue evaluating the efficacy of this therapy on Niemann Pick’s disease"
Fabry disease is characterized by a deficiency in the enzyme alpha-galactosidase, which results in reduced metabolism of globotriaosylceramide (GL-3). This can lead to the accumulation of GL-3 in the blood vessels of the kidneys, heart, brain, and skin, potentially causing stroke, heart attack, and kidney failure.
The trial enrolled 58 patients, with the control group receiving treatment every other week, and the experimental group receiving weekly treatments of enzyme-replacement therapy.
The study displayed that patients in the intervention arm had:
Reduced levels of GL-3 in their kidneys and heart
Normal GL-3 levels for 20 patients
Normal GL-3 accumulation in other organ systems, including the skin
These findings provide a path forward for patients stifled by the harsh realities of Fabry disease and other metabolic disorders.
Icahn School of Medicine Announces Breakthrough Enzyme Replacement Therapy Treatment for Fabry Disease
The treatment showed significant reductions in GL-3 accumulations, and is expected to be a leap forward for individuals who have malfunctioning enzymes as a result of Fabry disease
ABOUT ICAHN SCHOOL OF MEDICINE
Located in Manhattan, Mount Sinai School of Medicine strives to serve our East Harlem neighbours by strengthening health care delivery, improving quality of life, and providing educational opportunities. We are global leaders in clinical, basic science research, and medical education. Since 2000, we have secured over $168 million in public and private research funding, and rank #22 out of 125 medical schools in total research funding. For more information please visit https://icahn.mssm.edu/
Media Pitch
A reporter for STAT News with a doctorate from Stanford, Jonathan is a life science expert covering Biotech & Pharma and has previously covered rare disease therapies.
Jonathan Wosen
Hi Jonathan,
In alignment with your previous coverage of rare disease research, I thought you might be interested in a first look at topline results from Icahn School of Medicine's Phase 3 clinical study of an enzyme replacement therapy treatment to combat Fabry disease.
Icahn School of Medicine is planning to announce positive Phase 3 data of its investigative enzyme replacement therapy based on their technology in Niemann-Pick disease. The study enrolled 58 trial participants, with the experimental group receiving biweekly treatments of enzyme-replacement therapy. Coordinated by Dr. Robert J. Desnick and a team of researchers at Mount Sinai's East Harlem hospital, the study is the first of its kind in cohort size for Fabry disease.
In a high-need market with limited options the enzyme therapy marks a turning point in the fight against Fabry disease. The treatment's mechanism of action reduces GL-3 levels in vital organs to combat metabolic dysfunction.
Looking to the future, Icahn School of Medicine has initiated the FDA approval process to systemize a potential path to commercialization for a patient population with limited options.
I have attached the press release for more details. Feel free to reach out if this topic is of interest.
Thanks,
Rhiju Chakraborty
Account Executive, CG Life
rchakraborty@cglife.com
EXCLUSIVE: Phase 3 Results Show Icahn School of Medicine Enzyme Replacement Therapy Lowers GL-3 Levels